Au service
des femmes
depuis 1997
NOUVEAU
Découvrez LE KIT D’AUTO-DÉPISTAGE HPV / IST 4Gyn Platinium 7MED.
À réaliser lors de la mise en place d’un DIU, ce test est conçu pour évaluer et écarter les risques infectieux de types HPV et IST. Pensé pour le confort de toutes, le kit d’auto-dépistage 4Gyn Platinium 7MED est à réaliser chez soi en toute intimité, ou bien au cabinet d’un professionnel de santé.

Le laboratoire 7med
Le LABORATOIRE 7MED est une société française spécialisée dans la contraception féminine sans hormones. Nous concevons, fabriquons et commercialisons des dispositifs intra-utérins (DIU) au cuivre, plus connus sous le nom de « stérilets ».
Nos DIUs sont des Dispositifs Médicaux marqués CE 0459. Ils sont 100% produits en France, près de Vichy, en Auvergne Rhône-Alpes.
Le LABORATOIRE 7MED propose une gamme complète, pensée pour le confort de toutes. Nos différents modèles de DIU au cuivre visent à apporter une solution adaptée aux besoins de chacune.
Pour en savoir plus sur nous et nos valeurs, c’est par ici :
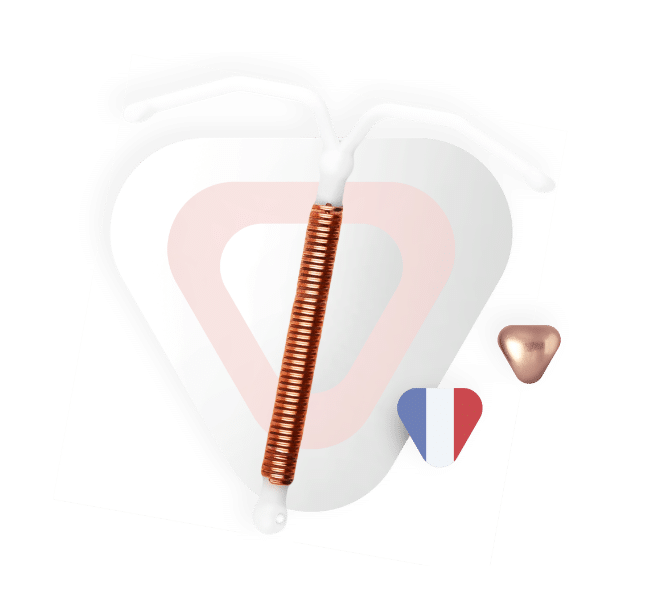
Le DIU au cuivre
Le saviez-vous ? Le DIU est la seconde méthode de contraception la plus utilisée en France après la pilule.
Préconisé en première intention par la Haute Autorité de Santé (HAS) chez les femmes en âge de procréer, le DIU au cuivre reste cependant l’objet de nombreuses idées reçues, notamment chez la femme nullipare (n’ayant jamais accouché).
Plus concrètement, qu’est-ce qu’un DIU ? Comment s’effectue la pose ? Est-ce une méthode adaptée à toutes ?
Le LABORATOIRE 7MED répond à vos questions :

Espace professionnels de santé

Depuis 2016, nous accompagnons les professionnels de santé dans la pose de DIU, grâce à un concept unique en France : l’ACADÉMIE 7MED.
Les formations de l’ACADÉMIE 7MED s’adressent aux gynécologues, aux médecins, aux sages-femmes… Composées de modules théoriques et pratiques, ces sessions sont dispensées par l’un de nos experts confirmés, en congrès ou au cabinet.
Vous êtes professionnel de santé ?
Rendez-vous dans votre espace pour en savoir plus :
à votre écoute
Une question, une suggestion ?
Le LABORATOIRE 7MED est à votre écoute !
N’hésitez pas à composer notre numéro vert : 0 800 940 777,
ou à prendre contact avec nous :

Espace
Professionnels
de Santé
